Description
Panbio Rapid Antigen Test (Expire Date: 07/12/2025)
For Healthcare Professionals (NOT for Home use) - For alternatives, please see Covid Rapid Self-Test.
Abbott Panbio Rapid Antigen Test device is an In Vitro Diagnostic Rapid Antigen Test for qualitative detection of SARS-CoV-2 Antigen (Ag) directed for Healthcare Professionals. Please check TGA Purchase Eligibility below where qualification form and training certificate must be submitted upon purchase.
Abbott Panbio COVID-19 Rapid Antigen Test device can identify potentially COVID-19 contagious patients with or without symptoms in 15 minutes to reduce virus spread. Abbott Panbio COVID-19 Rapid Test is recommended as a Point of Care Test (POCT) and, as such, has to be performed by a healthcare professional/ provider or someone who has received appropriate training (please refer to the "Purchase Eligibility" session below).
Test Accuracy - Very high sensitivity:
- Sensitivity: 98.1%
- Specificity: 99.4%
Benefits:
- Patient-friendly nasal self-collected swab option minimises health worker exposure.
- Accessible, affordable, easy to deploy, and provides quick, reliable.
- Fast identification of potentially contagious individuals
- Test results in 15 minutes
- Deploy at large scale at point of care
- Can be used in a wide variety of non-laboratory settings
- No special/additional instruments required
- Self-contained tube with "break off" swab minimizes staff exposure
- Extraction tube is fully enclosed for disposal
Each Pack Contains:
- 25 Test devices with desiccant in individual foil pouch
- Buffer (1 x 9 ml/bottle)
- 25 Extraction tubes
- 25 Extraction tube caps
- 1 Positive control swab
- 1 Negative control swab
- 25 Sterilized nasal swabs for sample collection
- 1 Tube rack
- 1 Quick Reference Guide
- 1 Instructions for use Materials Required but not Provided
Specifications:
- Test Time Results: 15 Minutes
- Storage: 2°C–30°C
- Sample Type: Nasal Swab
- TGA Registration: 345192
- CE marked
Ingredients of Main Components:
- 1 Test device Gold conjugate: Human IgG specific to SARS-CoV-2 Ag gold colloid and Chicken IgY - gold colloid, Test line: Mouse monoclonal antiSARS-CoV-2, Control line: Mouse monoclonal anti-Chicken IgY
- Buffer Tricine, Sodium Chloride, Tween 20, Sodium Azide (<0.1%), Proclin 300 Storage and Stability
Storage:
1. The Abbott Panbio Rapid Antigen Test Kit should be stored at a temperature between 2-30 °C. Do not freeze the kit or its components. Note: When stored in a refrigerator, all kit components must be brought to room temperature (15-30 °C) for a minimum of 30 minutes prior to performing the test. Do not open the pouch while components come to room temperature.
2. The Buffer bottle may be opened and resealed for each assay. The Buffer cap should be firmly sealed between each use. The Buffer is stable until expiration date if kept at 2-30 °C.
Instructions of Use:
- Perform the test immediately after removing the test device from the foil pouch.
- Do not use the test kit beyond its expiration date.
- The shelf life of the kit is as indicated on the outer package.
- Do not use the test kit if the pouch is damaged or the seal is broken.
- Direct swab specimens should be tested immediately after collection. If immediate testing is not possible, the swab specimen can be kept in an extraction tube filled with extraction buffer (300 μl) at room temperature (15-30 °C) for up to two hours prior to testing.
- For more technical information, please refer to the Instructions for Use Manual (IFU).
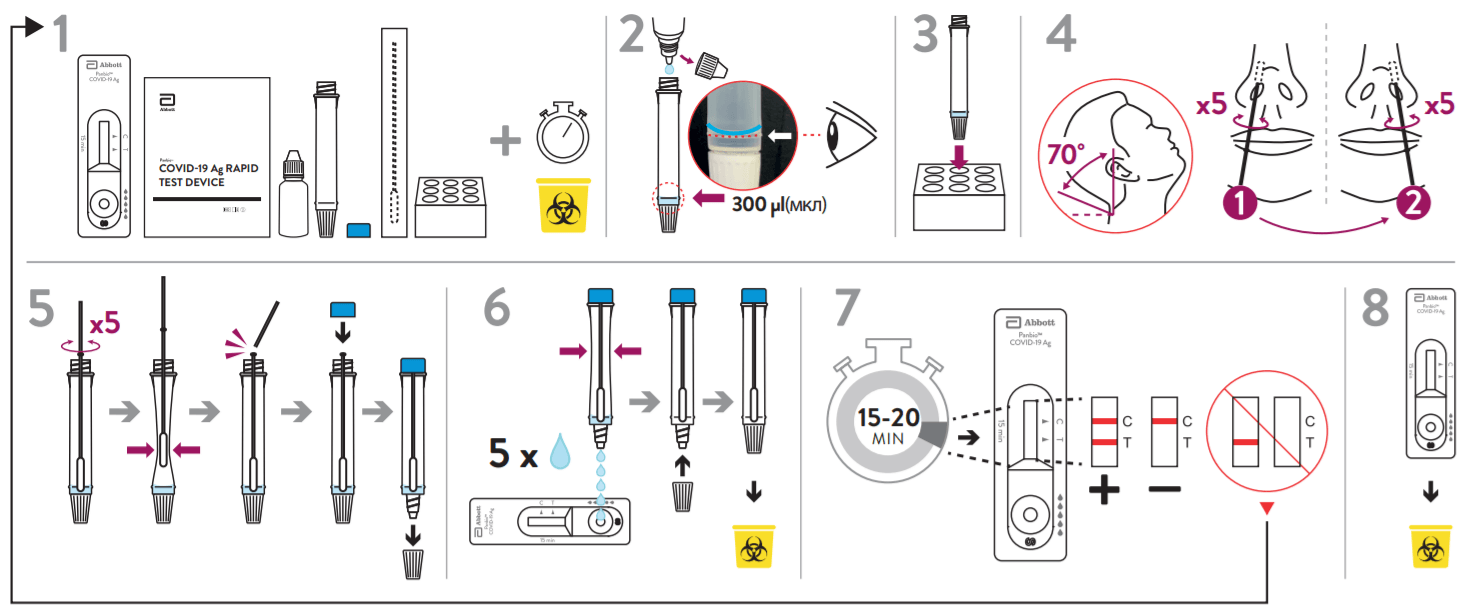
Materials required but not provided:
- Use of Personal Protective Equipment per local recommendations such as: Gown, Face Mask, Face Shield/Eye Goggles and Gloves
- Timer
- Biohazard Disposal Container
Management of Results:
Positive:
- A person who receives a positive rapid antigen test result needs to have an urgent PCR test on a second collection to determine whether COVID-19 is in fact present.
- When a person has a positive rapid antigen test result, they must be notified immediately to the NSW Health Public Health Unit (PHU) on 1300 066 055.
- The PHU will advise on safe transport to a local COVID-19 testing facility where they will have priority access to a PCR test.
- The person with a positive rapid antigen test result must remain in isolation until a definitive result is available. People with a confirmed positive test will have their results reported immediately to the PHU in line with high-risk results procedures.
Negative:
Continue to follow the latest health advice and restrictions in your area. If individuals develop any symptoms, even if mild, they must immediately get a standard COVID-19 test and isolate until they get a negative result from NSW Health.
Warnings:
- For in vitro diagnostic use only. Do not reuse the test device and kit components.
- These instructions must be strictly followed by a trained healthcare professional to achieve accurate results. All users have to read the instruction prior to performing a test.
- Do not eat or smoke while handling specimens.
- Wear protective gloves while handling specimens and wash hands thoroughly afterwards.
- Avoid splashing or aerosol formation of specimen and buffer.
- Clean up spills thoroughly using an appropriate disinfectant.
- Decontaminate and dispose of all specimens, reaction kits and potentially contaminated materials (i.e. swab, extraction tube, test device) in a biohazard container as if they were infectious waste and dispose according to applicable local regulations.
- Do not mix or interchange different specimens.
- Do not mix reagent of different lots or those for other products.
- Do not store the test kit in direct sunlight.
- To avoid contamination, do not touch the head of provided swab when opening the swab pouch.
- The sterilized swabs should be used only for nasal specimen collection.
- To avoid cross-contamination, do not reuse the sterilized swabs for specimen collection.
- Do not dilute the collected swab with any solution except for the provided extraction buffer.
- The buffer contains <0.1% sodium azide as a preservative which may be toxic if ingested. When disposed of through a sink, flush with a large volume of water.
- Do not use the positive or negative control swab for specimen collection.
Supporting Documentation:
Purchase Eligibility:
To be eligible to purchase the Panbio Antigen Rapid Test For Healthcare Professionals, please:
- Complete the Qualification for Supply COVID-19 Test Form available HERE.
- Return Form to info@alphamedicalsolutions.com.au for approval by Abbott, the manufacturer;
- Abbott is going to contact you directly to supervise the training session available on: Panbio COVID-19 Rapid Antigen Test Device - Customer Training Presentation (Voice Recorded)









